
On Aug. 15th, Cell Metabolism published a study led by Hui-Kuan Lin, PhD, that offers novel paradigms and strategies for targeting cancers and immunotherapy resistance.
The study, titled “NSUN2 is a Glucose Sensor Suppressing cGAS/STING to Maintain Tumorigenesis and Immunotherapy Resistance,” revealed an unprecedented finding: that glucose serves as a signaling molecule that binds directly to RNA methyltransferase NSUN2, a direct glucose sensor whose methyltransferase activity can be directly induced by glucose independently of its classic metabolism. DNA methyltransferases (MTases), are a family of enzymes that greatly impact gene regulation by catalyzing the methylation of DNA.
The study significantly expands our current understanding of how glucose can also orchestrate oncogenic processes. It outlines how glucose can act as signaling molecule independently of its metabolism by directly binding to and activating NSUN2 for maintaining TREX2 expression. This results in cGAS/STING repression to promote oncogenic processes and immunotherapy resistance. The cGAS-STING pathway regulates various aspects of the Cancer-Immunity Cycle (CIC).
Oncogenesis is the complex, multi-step process by which normal cells turn into cancerous cells, leading to cancer growth in the body. It involves genetic changes in a group of cells that causes them to grow and behave abnormally.
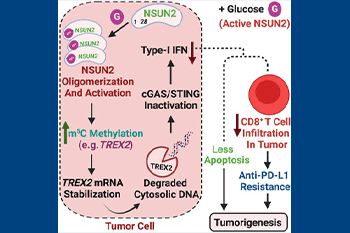
By targeting glucose/NSUN2/TREX2 axis, the study offers a potential strategy to target cancer without impacting normal glucose metabolism.
Developing immunotherapy agents by targeting Programmed death-1 (PD-1) or Programmed Death-Ligand 1 (PD-L1) has revolutionized therapy for cancer treatment in recent years. PD-1 is a cell surface receptor that functions as a T cell checkpoint and plays a central role in regulating T cell exhaustion. PD-L1 is a protein that can be found on the surface of many cells throughout the body. Many tumor cells have large amounts of PD-L1 that help the tumor cells evade the body's immune system.
However, the mechanisms underlying anti-PD-1/anti-PD-L1 immunotherapy resistance have not been well understood. Working in the Lin Lab, postdoctoral fellow Tingjin Chen, PhD, the paper’s first author, identifies the glucose/NSUN2/TREX2 axis as a key mechanism to drive the formation of cold tumors – those that show signs of inflammation- and their resistance to anti-PD-L1 immunotherapy.
The study provides evidence that targeting glucose/NSUN2/TREX2 axis is a promising strategy for cancer targeting and overcoming anti-PD-1/anti-PD-L1 immunotherapy resistance in cold tumors, commonly compared to impregnable fortresses surrounded by a moat. There are few T-cells in their "walls", and it is extremely difficult for them to mobilize an immune response.
The study offers a premise of converting cold tumors such as prostate cancer to hot tumors that can then respond to anti-PD-1/PD-L1 immunotherapy.
Lin joined the Duke Pathology faculty in May of 2023, as a professor and director of Prostate Cancer Research. Read the study Cell Metabolism here. Read his additional publications here.