Research Interests
Research interests in the Staats’ lab currently focus on:
Identifying and Characterizing Novel Mucosal Adjuvants and Their Mechanism of Action
Adjuvants are substances commonly added to vaccines that enhance the induction of protective immune responses to the vaccine antigen. We have been successful at identifying substances with mucosal adjuvant activity such as the pro-inflammatory cytokine interleukin 1α/β (IL-1α/β). IL-1α/β provides effective nasal adjuvant activity in mice, rabbits and non-human primates. Recent studies performed in collaboration with Dr. Soman Abraham have determined that the chemical mast cell activator compound 48/80 provides effective nasal adjuvant activity in mice and rabbits. Our lab continues to search for compounds that provide safe and effective nasal adjuvant activity.
Recent studies in the lab have focused on the mechanism of action of IL-1α when used as a nasal vaccine adjuvant. IL-1α/β is a pro-inflammatory cytokine and we expected that its adjuvant mechanism of action was related to its ability to induce local production of pro-inflammatory cytokines that subsequently activated dendritic cells for enhanced antigen presentation activity. However, our studies determined that nasal IL-1α must directly activate CD11c+ dendritic cells for maximal adjuvant activity while IL-1α/β-induced cytokine responses (serum and nasal wash) were not required for maximal adjuvant activity after nasal delivery of IL-1α.
Optimizing Nasal Immunization for Use in Humans
Nasal immunization studies in mice have demonstrated the ability of nasal immunization to induce protective immune responses equal to those induced by a vaccine delivered with a needle. However, when nasal immunization is performed in rabbits or non-human primates, animals with a nasal cavity structure/anatomy that closely resembles the human nasal cavity, nasal immunization is often not as effective as immunization delivered with a needle. Studies in our lab have demonstrated that an increased nasal residence time in rabbits correlates with increased vaccine immunogenicity. Studies are being performed to develop vaccine delivery techniques and vaccine formulations that maximize nasal residence time and therefore, the immunogenicity of the vaccine.
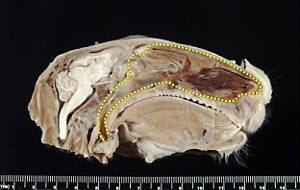
The nasal cavity of a rabbit is similar to the nasal cavity of humans and therefore the rabbit is a helpful animal model to optimize delivery of nasal vaccines.
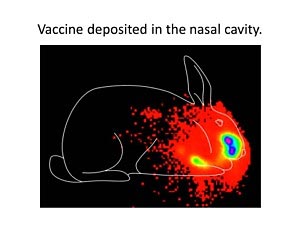
Gamma scintigraphy (performed in collaboration with Dr. W. Michael Foster) was used to track nasal delivery of liquid vaccine formulations. At the time of delivery, the vaccine formulation is predominantly in the nasal cavity.
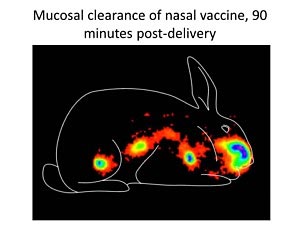
Gamma scintigraphy image at 90 minutes after nasal delivery demonstrating vaccine clearance from the nasal cavity and appearance in the gastrointestinal tract. Imaging technology is used to evaluate nasal vaccine formulations to identify formulations that maximize vaccine retention in the nasal cavity.
Evaluating Factors that Influence the Induction of Food Allergy and Developing Novel Mucosal Treatments for Food Allergy
The number of individuals with food allergy in steadily increasing in developed countries. The administration of food allergens via mucosal routes, a procedure known as “mucosal immunotherapy,” has provided encouraging results suggesting that mucosal immunotherapy is able to modify the host anti-food allergen response to reduce the severity of allergic responses. A new avenue of research in the laboratory is to 1) develop novel mucosal immunotherapy formulations (in collaboration with Dr. Wesley Burks, UNC Chapel Hill) and 2) evaluate the influence of environmental factors on the induction and severity of food allergies.
Publications
Please see Pub Med for current listings.
Book Sections
- Hickey, AJ, Staats, H, Roy, CJ, Powell, KG, Sullivan, V, Rothrock, G, & Sayes, CM. (2014, January 1). Nasal dry powder vaccine delivery technology. In Molecular Vaccines: From Prophylaxis to Therapy - Volume 2 (pp. 717-726)
Conference Papers
- Choi, HW, Brooking, R, Neupane, S, Lee, C-J, Miao, E, Staats, HF, & Abraham, SN. (2014, February). Salmonella Typhimurium Impedes Innate Immunity With a Mast Cell-Suppressing Tyrosine Phosphatase Sptp. Link to Item
- Johnson, BT, Kulis, M, Abraham, SN, Burks, AW, & Staats, HF. (2012, February). Nasal Immunization with Peanut Antigen and The Cationic Peptide Adjuvant Mastoparan 7 Induces Serum Humoral Immunity That Protects Peanut Allergic Mice Against Systemic Anaphylaxis. Link to Item
- Hofmann, AM, Jin, C, Staats, HF, & Abraham, SN. (2010, February). An In Vitro Model of Mast Cell Desensitization. Link to Item
- Hofmann, AM, Staats, HF, & Abraham, SN. (2009, December). Mast cell activator as a mucosal adjuvant in intranasal pertussis vaccine. Link to Item
- Pons, L, Buchanan, AD, Steele, PH, Staats, HF, & Burks, AW. (2006, February).CD4+CD25(high) T regulatory cells in egg-allergic children undergoing oral desensitization. Full Text Link to Item
- Kadono, T, Staats, HF, Steeber, DA, Tedder, TF, & Tamaki, K. (2004, March). Cutaneous and nasal immunity is L-selectin-dependent, whereas cooperation between L-selectin and beta 7 integrin is critical for intestinal immunity. Link to Item
- Peacock, JW, Nordone, SK, Jackson, S, Liao, HX, Letvin, N, Yafal, AG, Gritz, L, Mazzara, G, Haynes, BF, & Staats, HF. (2003, April 14). Immunization that induces maximal HIV-specific cell-mediated immunity in the reproductive tract and colon of female mice is not optimal in male. Link to Item
- Steeber, DA, Kadono, T, Staats, HF, Venturi, GM, Andrews, MC, Wagner, N, & Tedder, TF. (2003, April 14). Differential requirement for L-selectin- and beta 7 integrin-dependent lymphocyte migration in generation of gut- and nasal-associated mucosal immune responses. Link to Item
Journal Articles
- Johnson-Weaver, BT, McRitchie, S, Mercier, KA, Pathmasiri, W, Sumner, SJ, Chan, C, Germolec, D, Kulis, M, Burks, AW, & Staats, HF. (2018, February). Effect of endotoxin and alum adjuvant vaccine on peanut allergy. The Journal of allergy and clinical immunology, 141 (2), 791-794.e8
- Staats, HF, & Burkhart, DJ. (2018, January). Vaccine adjuvants: Softness makes it better. Nature materials, 17 (2), 113-114
- Norton, JN, Reynolds, RP, Chan, C, Valdivia, RH, & Staats, HF. (2017, September). Assessing the satisfaction and burden within an academic animal care and use program. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 31 (9), 3913-3921
- Jones, DI, McGee, CE, Sample, CJ, Sempowski, GD, Pickup, DJ, & Staats, HF. (2016, July). Modified Vaccinia Ankara Virus Vaccination Provides Long-Term Protection against Nasal Rabbitpox Virus Challenge. Clinical and vaccine immunology : CVI, 23 (7), 648-651
- Nelson, CS, Pollara, J, Kunz, EL, Jeffries, TL, Duffy, R, Beck, C, Stamper, L, Wang, M, Shen, X, Pickup, DJ, Staats, HF, Hudgens, MG, Kepler, TB, Montefiori, DC, Moody, MA, Tomaras, GD, Liao, H-X, Haynes, BF, Ferrari, G, Fouda, GGA, & Permar, SR. (2016, May). Combined HIV-1 Envelope Systemic and Mucosal Immunization of Lactating Rhesus Monkeys Induces a Robust Immunoglobulin A Isotype B Cell Response in Breast Milk. Journal of virology, 90 (10), 4951-4965
- Samo, M, Choudhary, NR, Riebe, KJ, Shterev, I, Staats, HF, Sempowski, GD, & Leduc, I. (2016, February). Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface. Vaccine, 34 (9), 1193-1200
- Phua, KKL, Staats, HF, Nair, SK, & Leong, KW. (2015, September). Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Journal of controlled release : official journal of the Controlled Release Society, 213, e66-e67
- Roggelin, L, Vinnemeier, CD, Fischer-Herr, J, Johnson-Weaver, BT, Rolling, T, Burchard, GD, Staats, HF, & Cramer, JP. (2015, August). Serological response following re-vaccination with Salmonella typhi Vi-capsular polysaccharide vaccines in healthy adult travellers. Vaccine, 33 (33), 4141-4145
- Bento, D, Staats, HF, & Borges, O. (2015, July). Effect of particulate adjuvant on the anthrax protective antigen dose required for effective nasal vaccination. Vaccine, 33 (31), 3609-3613