Research Interests
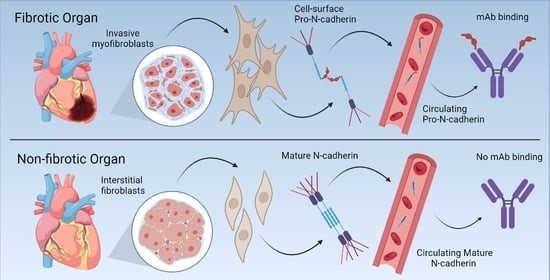
The Pizzo laboratory has a long history of studying aberrantly localized proteins and the role of dysregulated localization and protein processing in human disease. Recently, we discovered that the proprotein form of N-Cadherin (Pro-N-Cadherin; PNC) is aberrantly expressed on the surface of cells in pathological tissues, but not cells in healthy tissue. Pro-N-cadherin is normally processed by healthy cells in the trans-Golgi network (TGN) by proprotein convertase proteases to produce the mature form of N-cadherin, where the pro domain is cleaved from the protein, and the protein is subsequently transported to the cell surface to serve as a cell adhesion molecule (1). The normal rate of proteolysis and excision of the pro domain from N-cadherin within normal tissues leaves it undetectable without enrichment, and non-existent at the cellular surface of normal cells. However, there have been reports in the cancer biology literature demonstrating the presence of pro-N-cadherin on the surface of malignant cells and corresponding positive correlation with invasiveness of cancer cells expressing cell surface PNC (2, 3). Initial studies in collaboration with the Bachelder laboratory investigated this phenomenon in cancer, while our recent efforts have been focused on the role of cell-surface PNC in the setting of fibrosis.
We have recently published work in which we found that N-cadherin protein processing is involved in pathological fibrosis (4). Diseased tissues associated with fibrosis of the heart, lung, and liver were probed for PNC by immunohistochemistry and compared to healthy tissues. Myofibroblast cell lines were analyzed for cell surface PNC by flow cytometry and immunofluorescent microscopy. Soluble PNC products were immunoprecipitated from patient plasmas and an enzyme-linked immunoassay was developed for quantification. All fibrotic tissues examined show aberrant PNC localization. Cell surface PNC is expressed in myofibroblast cell lines isolated from cardiomyopathy and idiopathic pulmonary fibrosis but not on myofibroblasts isolated from healthy tissues. PNC is elevated in the plasma of patients with cardiomyopathy (p ≤ 0.0001), idiopathic pulmonary fibrosis (p ≤ 0.05), and nonalcoholic fatty liver disease with cirrhosis (p ≤ 0.05).
We have humanized a murine antibody and have shown that it significantly inhibits migration of PNC expressing myofibroblasts.
Ongoing research in the laboratory seeks to further understand the unique role of PNC in the pathogenesis and progression of fibrosis, develop specific and non-toxic PNC-directed therapeutics, and characterize the nature of PNC as a biomarker of human disease.
- Wahl, J. K., Kim, Y. J., Cullen, J. M., Johnson, K. R. & Wheelock, M. J. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J. Biol. Chem. 278, 17269–17276 (2003).
- Maret, D. et al. Surface expression of precursor N-cadherin promotes tumor cell invasion. Neoplasia 12, 1066–1080 (2010).
- Nelson, E. R. et al. Chemotherapy enriches for an invasive triple-negative breast tumor cell subpopulation expressing a precursor form of N-cadherin on the cell surface. Oncotarget 7, 84030–84042 (2016).
- Ferrell, P.D.; Oristian, K.M.; Cockrell, E.; Pizzo, S.V. Pathologic Proteolytic Processing of N-Cadherin as a Marker of Human Fibrotic Disease. Cells 2022, 11, 156. https://doi.org/10.3390/cells11010156
Publications
Please see Pub Med for current listings.
Conference Papers
- Mo, L, Kennedy, M, Berchuck, A, Cianciolo, G, Bachelder, RE, and Pizzo, SV. "Abstract POSTER-THER-1420: Ascites drives ovarian cancer stem-like cell growth: therapeutic opportunities." August 15, 2015
- Allott, EH, Masko, EM, Choy, A, Gaines, AR, Solomon, KR, Pizzo, SV, and Freedland, SJ. "Abstract 3698: Inhibition of cholesterol uptake with ezetimibe reduces intra-tumoral testosterone levels and slows tumor growth in transgenic mouse models of prostate cancer." April 15, 2013
- Masko, EM, Solomon, KR, Valilis, NA, Gaines, AR, Muehlbauer, MJ, Newgard, CB, Dewhirst, MW, Pizzo, SV, and Freedland, SJ. "THE EFFECTS OF CHOLESTEROL TREATMENT DRUGS ALONE AND IN COMBINATION ON PROSTATE TUMOR XENOGRAFT GROWTH." April 2012
Journal Articles
- Al-Hashimi, AA, Lebeau, P, Majeed, F, Polena, E, Lhotak, Š, Collins, CAF, Pinthus, JH, Gonzalez-Gronow, M, Hoogenes, J, Pizzo, SV, Crowther, M, Kapoor, A, Rak, J, Gyulay, G, D'Angelo, S, Marchiò, S, Pasqualini, R, Arap, W, Shayegan, B, and Austin, RC. "Autoantibodies against the cell surface-associated chaperone GRP78 stimulate tumor growth via tissue factor." The Journal of biological chemistry 292, no. 51 (December 2017): 21180-21192
- Gopal, U, and Pizzo, SV. "Cell surface GRP78 promotes tumor cell histone acetylation through metabolic reprogramming: a mechanism which modulates the Warburg effect." Oncotarget 8, no. 64 (December 2017): 107947-107963
- Gonzalez-Gronow, M, Fiedler, JL, Farias Gomez, C, Wang, F, Ray, R, Ferrell, PD, and Pizzo, SV. "Myelin basic protein stimulates plasminogen activation via tissue plasminogen activator following binding to independent l-lysine-containing domains." Biochemical and biophysical research communications 490, no. 3 (August 2017): 855-860
- Masko, EM, Alfaqih, MA, Solomon, KR, Barry, WT, Newgard, CB, Muehlbauer, MJ, Valilis, NA, Phillips, TE, Poulton, SH, Freedland, AR, Sun, S, Dambal, SK, Sanders, SE, Macias, E, Freeman, MR, Dewhirst, MW, Pizzo, SV, and Freedland, SJ. "Evidence for Feedback Regulation Following Cholesterol Lowering Therapy in a Prostate Cancer Xenograft Model." The Prostate 77, no. 5 (April 2017): 446-457
- Jensen, BC, Bultman, SJ, Holley, D, Tang, W, de Ridder, G, Pizzo, S, Bowles, D, and Willis, MS. "Upregulation of autophagy genes and the unfolded protein response in human heart failure." International Journal of Clinical and Experimental Medicine 10, no. 1 (January 30, 2017): 1051-1058
- Nelson, ER, Li, S, Kennedy, M, Payne, S, Kilibarda, K, Groth, J, Bowie, M, Parilla-Castellar, E, de Ridder, G, Marcom, PK, Lyes, M, Peterson, BL, Cook, M, Pizzo, SV, McDonnell, DP, and Bachelder, RE. "Chemotherapy enriches for an invasive triple-negative breast tumor cell subpopulation expressing a precursor form of N-cadherin on the cell surface." Oncotarget 7, no. 51 (December 2016): 84030-84042
- Bultman, SJ, Holley, DW, G de Ridder, G, Pizzo, SV, Sidorova, TN, Murray, KT, Jensen, BC, Wang, Z, Bevilacqua, A, Chen, X, Quintana, MT, Tannu, M, Rosson, GB, Pandya, K, and Willis, MS. "BRG1 and BRM SWI/SNF ATPases redundantly maintain cardiomyocyte homeostasis by regulating cardiomyocyte mitophagy and mitochondrial dynamics in vivo."Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology25, no. 3 (May 2016): 258-269.
- Gopal, U, Gonzalez-Gronow, M, and Pizzo, SV. "Activated α2-Macroglobulin Regulates Transcriptional Activation of c-MYC Target Genes through Cell Surface GRP78 Protein."The Journal of biological chemistry 291, no. 20 (May 2016): 10904-10915.
- de Ridder, GG, Lundblad, RL, and Pizzo, SV. "Actions of thrombin in the interstitium."Journal of thrombosis and haemostasis : JTH 14, no. 1 (January 2016): 40-47. (Review)
Other Articles
- Fuchs, HE, and Pizzo, SV. "Catabolism of antithrombin III-protease complexes." Thromb Res 32, no. 2 (October 15, 1983): 253-254. (Letter) Link to Item