Research Interests
Mechanism of deregulated GTP metabolism in cancer cells
GTP-binding proteins (G proteins) regulate a vast variety of cellular processes and are frequently hyper-activated in human cancers. Enzymes controlling the de novo biosynthesis of GTP are also substantially deregulated during tumorigenesis and metastasis. Historically, changes in GTP levels were not considered as a regulatory step in activation of G-proteins in live cells. This is because average intracellular GTP concentration measured by HPLC or mass spectrometry (~500µM) is much higher than the GTP dissociation constant (Kd) of multiple G-proteins including small RHO-GTPases RAC1 or RHOA.
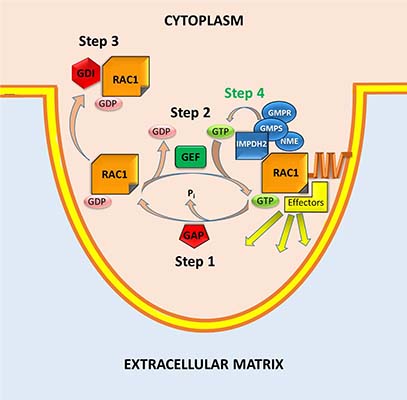
Up until now no methods existed to detect fluctuations of free GTP in live cells. We recently reported genetically encoded intracellular sensors of free GTP. (Bianchi-Smiraglia et al, Nature Methods, 2017) These sensors for the first time made possible visualization of free GTP changes in live cells and identified regions with low (~30µM) and high local GTP concentration.
Furthermore, by combining genetically encoded GTP biosensors and a RAC1 activity biosensor, we demonstrated that GTP levels fluctuating around RAC1-GTP Kd correlated with RAC1 activity in live cells. RAC1 colocalized in protrusions of invading cells with several guanylate metabolism enzymes, including rate-limiting inosine monophosphate dehydrogenase 2 (IMPDH2), GMPR, GMPS and NME (see a diagram). Substitution of endogenous IMPDH2 with IMPDH2 mutants incapable of binding RAC1 did not affect total intracellular GTP levels but suppressed RAC1 activity. Accordingly, targeting IMPDH2 away from the plasma membrane did not alter total intracellular GTP pools but decreased GTP levels in cell protrusions, RAC1 activity, and cell invasion. ( Bianchi-Smiraglia et al, Nature Communications, 2021) These findings represent a paradigm shift in the understanding of Rac1 regulation (Step 4 on the diagram). Currently we study mechanisms of intracellular GTP distribution and explore the anti-guanylate therapy as novel strategy for melanoma intervention.
Identification of polyamine and lipid metabolism targets in multiple myeloma
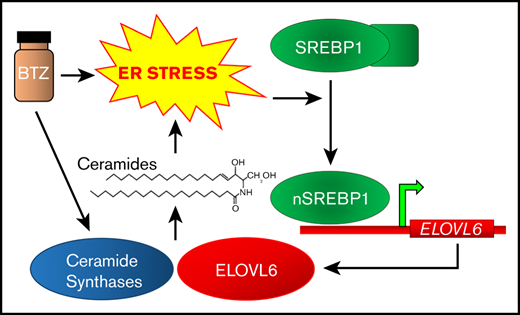
Multiple myeloma (MM) is a plasma cell disorder that accounts for approximately 10% of all hematologic malignancies. Due to high immunoglobulin production in endoplasmic reticulum (ER), MM cells continuously undergo ER stress. This feature makes MM susceptible to agents that exacerbate ER stress, such as proteasome inhibitor bortezomib. Yet currently MM is incurable for most patients due to rapidly developing resistance to proteasome inhibition. Recently by studying global metabolome and transcriptome in cultured and patient MM cells, we identified key enzymes involved in regulation of endoplasmic reticulum and oxidative stress (KLF9 and TXNRD2), polyamine metabolism (ODC1 and AZIN1), and fatty acid biosynthesis (fatty acid elongase ELOVL6, see a diagram) as major clinically relevant regulators of multiple myeloma resistance to bortezomib. (Fink et al, Leukemia; Fink et al, Cell Reports; Bianchi-Smiraglia et al, Journal of Clinical Investigation, 2018; Lipchick et al, Blood Adv, 2021) For some of the identified enzymes we uncovered mechanism of their involvement in control of MM cell viability, whereas the function of others is currently being pursued.
Exploring lineage-specific transcriptional regulators
Identification of lineage-specific transcription factors is important for understanding how the disease is manifested in different tissues. Of a particular interest are transcription factors oppositely regulating phenotypes in different lineages. In epithelial cells, E/N cadherin switch represents a hallmark of epithelial-mesenchymal transition (EMT), a process by which static cells acquire a mesenchymal-like phenotype, including migratory and invasive capabilities. In search for transcription factors regulating the EMT-like transition in melanocytic cells, we evaluated several bona fide regulators of EMT. One of them, FOXQ1, a member of FOX family of transcription factors, is overexpressed at advanced stages in several human carcinomas where it promotes E/N-cadherin switch ultimately leading to increased invasion.
Unexpectedly, we found that FOXQ1 levels are significantly lower in metastatic melanoma specimens compared to primary melanomas. Accordingly, we identified that in melanoma cells, FOXQ1 suppresses the same processes it activates in carcinoma cells: expression of N-cadherin gene (CDH2), EMT, invasion, and metastasis.
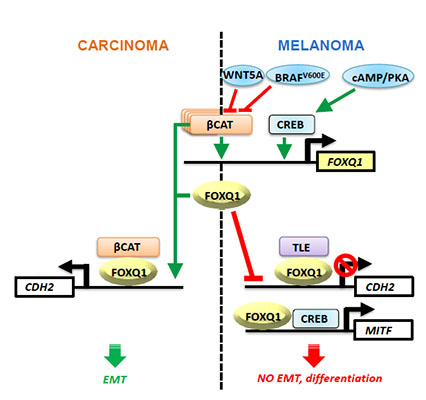
Mechanistically, we demonstrated that like LEF1/TCF4, FOXQ1 interacts with nuclear b-catenin and TLE (groucho) proteins and that the b-catenin/TLE ratio, which is higher in carcinoma than melanoma cells, determines the effect of FOXQ1 on N-cadherin gene transcription (see diagram). Accordingly, other FOXQ1-dependent phenotypes can be manipulated by altering nuclear ß-catenin or TLE proteins levels. (Bagati et al, Cell Reports, 2017)
Intriguingly, FOXQ1 is also involved in regulation of differentiation in normal melanocytes (Bagati et al, Cell Death and Differentiation, 2018) and keratinocytes via yet unknown mechanisms. Currently, we are establishing such mechanisms and in parallel pursuing the role of Foxq1 in skin biogenesis in mouse models.
Publications
Book Sections
- Oncogene-Induced Senescence. Methods and Protocols. Nikiforov MA (Editor). Methods in Molecular Biology. Springer. 2017; ISBN 978-1-4939-6670-7
Conference Papers
- 06.25.17: Gordon Research Conference. "Regulators of Polyamine Levels as Antineoplastic Drug Targets" Polyamine Metabolism in Disease and Polyamine-Targeted Therapies. Waterville Valley, NH, USA
- 06.23.19: Gordon Research Conference. "Role of Polyamines in Anticancer Drug Resistance". Polyamines in Cancer Biology, Inflammation, Microbiome, Plants and Pathogens. Waterville Valley, NH, USA
- 11.20.19: Society for Melanoma Research. “Leveraging GTP Metabolism For The Suppression of Melanoma Invasion and Drug Resistance”. Annual Congress, Salt Lake City, UT, USA
Journal Articles
-
David W. Wolff, Zhiyong Deng, Anna Bianchi-Smiraglia, Colleen E. Foley, Zhannan Han, Xingyou Wang, Shichen Shen, Masha M.Rosenberg, Sudha Moparthy, Dong Hyun Yun, Jialin Chen, Brian K. Baker, Matthew V.Roll, Andrew J.Magiera, Jun Li, Edward Hurley. Maria Laura Feltri, Anderson O. Cox, Jingyun Lee, Cristina M. Furdui, Liang Liu, Wiam Bshara, Leslie E.W. LaConte, Eugene S. Kandel, Elena B. Pasquale, Jun Qu, Lizbeth Hedstrom, Mikhail A.Nikiforov. Phosphorylation of guanosine monophosphate reductase triggers a GTP-dependent switch from pro- to anti-oncogenic function of EPHA4. Cell Chemical Biology, Published: February 10, 2022
- Bianchi-Smiraglia A, Rana MS, Foley CE, Paul LM, Lipchick BC, Hurley E, Affronti HC, Bakin AV, Smiraglia DJ, Feltri ML, Sousa R, Nikiforov MA. Generation and characterization of internally ratiometric fluorescent sensors for evaluation of intracellular GTP levels and distribution. Nature Methods 2017; 14:1003-1009.
- Bianchi-Smiraglia A, Wolff DW, Marston DJ, Deng Z, Moparthy S, Wombacher RM, Mussell AL, Shen S, Yun DH, Cox AO, Furdue C, Hurley E, Feltri ML, Qu J, Hollis T, Kengne JBN, Fongang B, Sousa RJ, Kandel ME, Kandel ES, Hahn KM, and Nikiforov MA. Regulation of local GTP availability controls RAC1 activity and cell invasion. Nature Communications 2021; 12:6091.
- Fink EE, Mannava S, Bagati A, Bianchi-Smiraglia A, Nair JR, Moparthy K, Lipchick BC, Drokov M, Utley A, Ross J, Mendeleeva LP, Savchenko VG, Lee KP, Nikiforov MA. Mitochondrial thioredoxin reductase regulates major cytotoxicity pathways of proteasome inhibitors in multiple myeloma cells. Leukemia 2016; 30:104-111.
- Fink EE, Moparthy S, Bagati A, Bianchi-Smiraglia A, Lipchick BC, Wolff DW, Roll MV, Wang J, Liu S, Bakin AV, Kandel ES, Lee AH, Nikiforov MA. XBP1s-KLF9 axis acts as a molecular rheostat to control the transition from adaptive to cytotoxic unfolded protein response. Cell Reports 2018; 25:212-223.
- Bianchi-Smiraglia A, Bagati A, Fink EE, Affronti HC, Lipchick BC, Moparthy S, Long MD, Rosariao SR, Lightman SM, Moparthy K, Wolff DW, Yun DH, Han Z, Polechetti A, Roll MV, Gitlin II, Leonova KI, Rowsam AM, Kandel ES, Gudkov AV, Bergsagel PL, Lee KP, Smiraglia DJ, Nikiforov MA. Inhibition of the Aryl Hydrocarbon Receptor - Polyamine Biosynthesis Axis Suppresses Multiple Myeloma. Journal of Clinical Investigations 2018; 128:4682-4696.
- Lipchick BC, Utley A, Han Z, Moparthy S, Yun DH, Bianchi-Smiraglia A, Wolff DW, Fink E, Liu L, Furdui CM, Lee J, Lee KP, Nikiforov MA. The fatty acid elongase ELOVL6 regulates bortezomib resistance in multiple myeloma. Blood Advances 2021; 5:1933-194
- Bagati A, Moparthy S, Bianchi-Smiraglia A, Lipchick BC, Polechetti A, Kolesnikova M, Jowdy P, Fink EE, Lyskawa A, Ross J, Wawrzyniak JA, Mahpour A, Wrazen B, Shafirstein B, Paragh G, Nemeth MJ, Nikiforov MA. Melanoma Suppressor Functions of the Carcinoma Oncogene FOXQ1. Cell Reports 2017; 20:2820-2832.
- Bagati A, Bianchi-Smiraglia A, Moparthy S, Kolesnikova K, Fink EE, Kolesnikova M, Roll MV, Jowdy P, Wolff DW, Polechetti A, Yun DH, Lipchick BC, Paul LM, Wrazen B, Moparthy K, Mudambi S, Morozevich GE, Georgieva SG, Wang J, Shafirstein G, Liu S, Kandel ES, Berman AE, Box NF, Paragh G, Nikiforov MA. FOXQ1 controls the induced differentiation of melanocytic cells. Cell Death and Differentiation 2018; 25:1040-1049.