Data at Their Digital Fingertips: Tissue-based Research and Precision Cancer Medicine Comes of Age
Ten years ago, visionaries in the Department of Pathology and Duke Cancer Institute (DCI) saw an opportunity to advance human tissue-based research and precision cancer medicine at Duke and grabbed it.

A working group led by Pathology faculty Rajesh Dash, MD (then co-leader with Alan Proia, MD, PhD, of the Duke Comprehensive Cancer Center Biorepository), Michael Datto, MD, PhD (then- leader of the Genome Trials Support Facility), and Shannon McCall, MD, together with other leaders in the Duke School of Medicine (SOM) began to discuss merging into one entity the Duke Comprehensive Cancer Center Biorepository, which had started to languish from under-funding, and the nascent Genome Trials Support Facility.
They had the ear of Michael Kastan, MD, PhD, the William and Jane Shingleton Distinguished Professor of Pharmacology and Cancer Biology and executive director of Duke Cancer Institute (DCI). Kastan, who had joined Duke in 2011, believed that DCI, the successor entity to the National Cancer Institute-designated Duke Comprehensive Cancer Center, could serve as a national model for structuring cancer programs — bringing together research, patient care, and education under one umbrella.
In 2012, DCI and the Duke University School of Medicine committed to a five-year, $3 million investment in a new Duke BioRepository & Precision Pathology Center (BRPC) — a clinical research and discovery resource with its administrative home in the Department of Pathology. Spurred by key investments in technology, services, and personnel, the BRPC grew, thrived, and progressively built a national reputation.

Datto, the inaugural director of the BRPC, was instrumental in designing its Institutional Review Board protocol. He modeled the BRPC after other Duke University Health System Clinical Laboratories and prepped the BRPC for accreditation by the College of American Pathologists (CAP).
In 2013, McCall was named director and the BRPC received CAP accreditation as a tissue, blood, and fluid biorepository, tissue procurement service, and research support core laboratory. In 2014, the BRPC became an approved Duke University School of Medicine Service Center (also known as a Core Research Facility) and Duke Cancer Institute Shared Resource.
By 2022, the number of BRPC personnel had expanded to 14 from just two in 2012. This included the addition, in 2019, of three subspecialized associate director roles: William Jeck, MD, PhD, for the Artificial Intelligence (AI) & Computational Pathology service; Jadee Neff, MD, PhD ,for the Genomics service and for the Digital Spatial Profiling service; and Avani Pendse, MD, PhD, for the Immunohistochemistry and Proteomics services. Not included in the 14 — a cadre of second-year Duke Pathologist Assistant students engaged in a work-study capacity at the BRPC and a regular rotation of DCI molecular oncology trainees shadowing pathologists in the “frozens lab.”

“The partnership between our department and DCI over the past decade has resulted in important advances whose impact on cancer research, the field of precision medicine, and patients is immeasurable,” says Chair of the Department of Pathology, Jiaoti Huang, MD, PhD.
He points out that the BRPC has served as the biospecimen/pathology core for multiple U.S. government-funded, multi-institutional, and homegrown studies.
Leading cancer pathologists have worked hand in glove with other DCI investigators on projects from immune profiling in gastric cancer (National Cancer Institute SPORE, cancer health disparities); to multi-institutional digital spatial profiling in breast pre-cancer cells (National Cancer Institute Breast Pre-Cancer Atlas); to digital spatial profiling in potentially pre-cancerous pancreatic lesions (Allen Pancreatic Cancer Research Lab) to the establishment of a Senescent Cell Human Tissue Mapping Center at Duke (National Institutes of Health Cellular Senescence Network) and more.
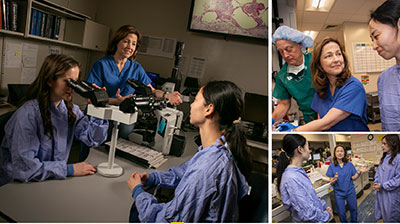
“From my perspective we’ve had a wonderful teaching experience,” said McCall at the time. “On this day in particular they got a chance to see how a surgeon (gynecologic surgical oncologist Andrew Berchuck, MD) needed a pathologist’s input in real time.”
With McCall as principal investigator, the BRPC has served as the base for the National Cancer Institute-supported Southern Division of the Cooperative Human Tissue Network since 2019.
The BRPC, DCI, and the DUHS Clinical Labs have advanced research and clinical practice through a multi-pronged Duke Precision Cancer Medicine Initiative; first with the coding and development, led by Datto, of an in-house Molecular Registry of Tumors (Frameshift MRT) database to organize and optimize previously accumulated and incoming tumor molecular sequencing records, and then the establishment of a multi-disciplinary Molecular Tumor Board — co-directed by oncologists John Strickler, MD, and Matthew McKinney, MD —to operationalize that data to inform treatment decisions.
Through this initiative, Duke Cancer Institute oncologists can identify whether specific molecular alterations in a patient’s cancer cells are potentially “druggable” with any existing targeted therapies (including in clinical trials) and whether these alterations are hereditary or tumor-specific. They can opt to receive “therapy alerts” from genetics scientist Michelle Green, PhD — Molecular Tumor Board senior research program leader, and main user and manager of Frameshift MRT, when a “hit” for their patients is detected. And their most complicated patient cases are reviewed, on a weekly basis, by the Molecular Tumor Board.
Per Green, the “hit rate” of matching patients to targeted therapies and the number of patients having a dramatic response to treatment continues to increase. Thanks in part to this precisely targeted, personalized medicine, DCI is helping patients in its catchment area and beyond to live longer and live better—turning cancer, for more and more patients with advanced disease, into a manageable chronic disease.

As of August 2022, Frameshift MRT contained comprehensive data from more than 9,000 tumor molecular testing reports for more than 8,000 patients, across more than 60 cancer types, as well as an additional 5,700-plus hereditary (germline) testing reports.
This year, three Duke Cancer Network sites in rural North Carolina gained streamlined access to molecular sequencing tests, the Molecular Registry of Tumors, and the Molecular Tumor Board at DCI, which has so far reviewed more than 50 DCN patient cases (mainly lung, colorectal, prostate, and breast cancers).

In a DCI Survivorship Day forum held this past June, faculty leaders were asked what major advancement had changed the face of cancer care in the past 50 years. Breast oncologist and professor Carey Anders, MD, interim chief of the Division of Medical Oncology, Department of Medicine, chose to highlight “tumor sequencing” for its capacity to identify molecular alterations that could be cancer’s “Achilles heels.”
“When I think back to nearly two decades ago, when I was in my training, the amount of information I had about an individual tumor was so limited compared to the information that I currently have in the clinic and that really comes from looking at the genes and the alterations in the genes in the tumor tissue as well as sometimes tumor cells circulating in the blood,” said Anders, who also serves as medical director of the Duke Center for Brain and Spine Metastasis at DCI. “Having that information in hand has been just extraordinary in terms of how we tailor our treatments to our patients. I've really loved the program that's been developed at Duke Cancer Institute. The Molecular Registry of Tumors really helps facilitate clinical care and research. I think we're getting much more precise (with targeted therapies). We’re more able to sustain responses to treatment over time.”
Investigators have used Frameshift MRT for 45 research projects and counting, including in colorectal, pancreatic, breast and prostate cancers. They’ve used Frameshift MRT to design clinical trials and search for trial candidates based on their tumor molecular profiles; answer research questions; and develop pre-clinical and retrospective research studies. Many of the studies are looking at associations between molecular alterations and patient outcomes.
At least one of the studies, led by Tomi Akinyemiju, PhD, MS, (DCI social and molecular cancer epidemiologist and associate director, Community Outreach, Engagement and Equity at DCI) is assessing inequities in genomic testing between races by linking and analyzing data from Frameshift MRT with data from the Duke Tumor Registry.
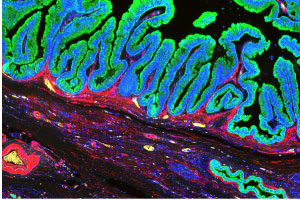
“An advantage of the Digital Spatial Profiling system is that it allows for the areas of high grade and low-grade dysplasia to be separately analyzed, giving a better understanding of what is truly driving these cysts towards malignancy," explains Eckhoff.
“One of the most extraordinary aspects of the BRPC is its commitment to collecting samples from patients of diverse racial and ethnic backgrounds,” says Steven Patierno, PhD, deputy director of Duke Cancer Institute, a professor in the departments of Medicine, Family Medicine and Community Health, Pharmacology and Cancer Biology, and principal investigator for an NCI SPORE grant on cancer health disparities. “Molecular analysis of tumor samples from a diversity of patients is crucial to equitable translation of cancer medicine. The Duke BRPC is at the forefront of being purposeful and deliberate in diversifying its biobank.”
For more than three years, DCI has been sharing its genomic data (de-identified) with an American Association for Cancer Research-run consortium of institutions — PROJECT GENIE (Genomics, Evidence, Neoplasia, Information, Exchange) — which pools the genomic data it receives into a national molecular registry of tumors to be used by researchers for the global good of cancer research and care. McCall is principal investigator.
Thousands of cancer patients have donated their blood, excess tissue, and biopsy samples to the BRPC — all in the name of and the belief in research toward cures for cancer – even though they may not benefit directly. These donations will continue to drive basic, translational, and clinical research forward.
“None of the advancements we’ve made and will make in the future would be possible without them, and we are eternally grateful,” says McCall.
“The BRPC has proven to be a wildly successful experiment in centralized biobanking, with benefits for Duke investigators and patients alike, and serves as a national model,” says Kastan.
“I am so proud of and grateful to Drs. Datto and McCall for their leadership of this effort and for the partnership of the Department of Pathology in making this a reality. I anticipate that the DCI/Pathology partnership will bring further growth and even greater impact in the years to come.”
Click here to learn how the Precision Cancer Medicine Initiative (PCMI) is advancing research, extending lives, and breaking down barriers to access.
Learn more about BRPC/DCI cancer research collaborations in "Partners to the Core."
Explore BRPC Milestones.
Click here to learn about new developments at the Clinical Molecular Diagnostics Laboratory (MDL).
